A transition metal is defined by IUPAC as "an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell”. Transition metals are found in Groups 4 through 11of the periodic table; elements in Groups 3 and 12 are sometimes included. Similar to the metals, transition metals are malleable and ductile, conduct heat and electricity, and form positive ions. However, these elements are more electronegative and more likely to form covalent compounds. Transition metals can form useful alloys with other transition or metallic elements.More
Note:The elements which are present in Red color box are Transition Metals.

| 1 H Hydrogen | 2 He Helium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 li lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
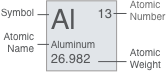
| 11 Na sodium | 12 Mg Magnesium | 13 Al Aluminium | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titanium | 23 V Vanadium | 24 Cr Cromium | 25 Mn Manganesse | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 Ar Arsenic | 34 Se Selanium | 35 Br Bromine | 36 Kr Krypton |
| 37 Rb Rubidium | 38 Sr Strontium | 39 Ca Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | 43 Tc Tecnetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadnium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 55 Cs Caesium | 56 Ba Barium | 57 la Lanthan... | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | 84 Po Polonnium | 85 At Astatine | 86 Rn Radon |
| 87 Fr Francium | 88 Ra Radium | 89 Ac Actinium | 104 Rf Rutherfo.. | 105 Db Dubnium | 106 Sg Seaborgium | 107 Bh Bohrium | 108 Hs Hassiumy | 109 Mt Meitnerium | 110 Ds Damstadium | 111 Rg Roentgenium | 112 Cn Copemicium | 113 Nh Nihonium | 114 Fl Flerovium | 115 Mc Moscovium | 116 Lv Livermorium | 117 Ts Tennessi.. | 118 Og Oganesson |
| 58 Ce Cerium | 59 Pr Praseodium | 60 Nd Neodymium | 61 Pm Promethium | 62 Sm Samarium | 63 Eu Europium | 64 Gd Gadolini.. | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Holmium | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | ||||
| 90 Th Thorium | 91 Pa Protactinium | 92 U Uranium | 93 Np Neptunium | 94 Pu Plutonium | 95 Am Americium | 96 Cm Curium | 97 Bk Berkelium | 98 Cf Californi.. | 99 Es Einstenium | 100 Fm Fermium | 101 Md Mendelevium | 102 No Nobelium | 103 Lr Lawrencium |
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They all can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ferromagnetic near room temperature are transition metals (iron, cobalt and nickel) or inner transition metals (gadolinium). English chemist Charles Rugeley Bury (1890–1968) first used the word transition in this context in 1921, when he referred to a transition series of elements during the change of an inner layer of electrons (for example n = 3 in the 4th row of the periodic table) from a stable group of 8 to one of 18, or from 18 to 32.[1][2][3] These elements are now known as the d-block.